(Photo Credits: SHVETS production from Pexels)
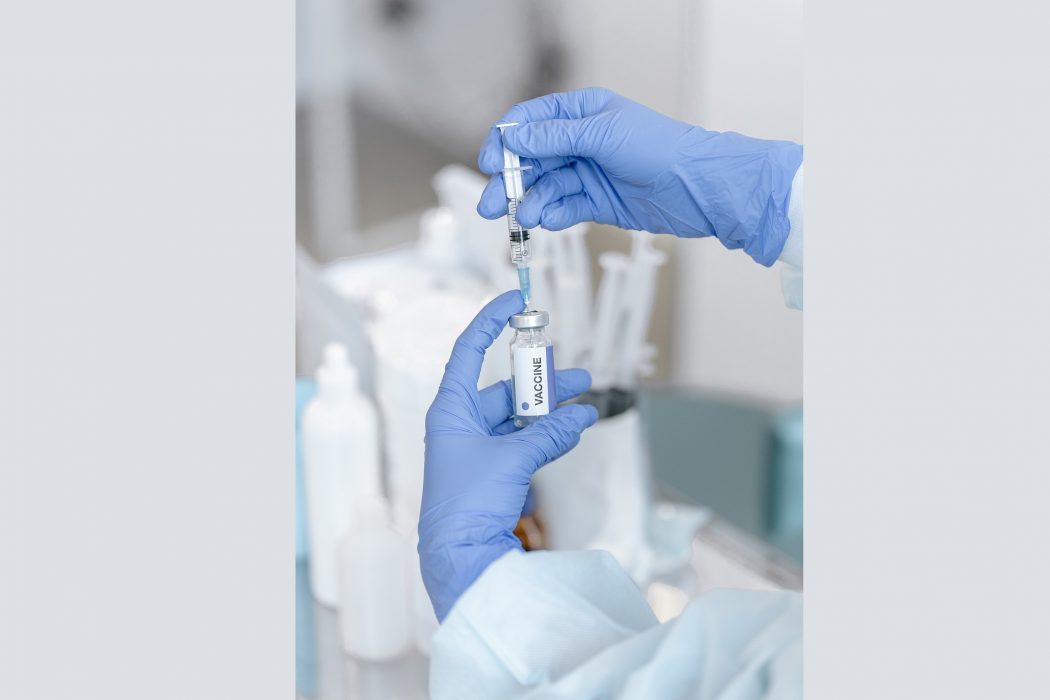
Moderna, a Massachusetts-based biotechnology company, is set to begin its clinical trials for their HIV vaccine this week, according to an information posted in the National Institutes of Health’s clinical trial registry. View it here.
The human trials—which will begin this August 19, 2021—will run through spring 2023. A total of 56 HIV-negative participants from ages 18 to 50 will be administered with one or two of Moderna’s HIV vaccine candidates: the mRNA-1644 and mRNA-1644v2-Core.
This is the Phase I of the study, and it will involve “test safety by administering different dose strengths and observing how the body and drug interact,” LGBTQNation reports. The Phase II of the study on the other hand, will “test the vaccine’s overall effectiveness,” while Phase III trials—the last one—will “compare the safety and effectiveness of the new vaccine against the current HIV prevention medications.”
mRNA Technology Explained
For their HIV vaccines, Moderna is using the messenger RNA (mRNA) technology which the company previously used in their COVID-19 vaccine. Centers for Disease Control and Prevention (CDC) explained that “mRNA vaccines are a new type of vaccine to protect against infectious diseases.”
The mRNA technology is said to have been around for 10 years though it was primarily used in immunotherapy cancer treatments. Pfizer-BioNTech and Moderna are the first to use mRNA technology for vaccines.
Further, CDC explained: “mRNA vaccines teach our cells how to make a protein—or even just a piece of a protein—that triggers an immune response inside our bodies.”
These proteins “train the immune system to fight off the virus.” Simply put, mRNA “teaches the body to recognize a virus” based on that protein so that once it encounters the virus, it will attack that virus.
More importantly, mRNA-based vaccines can be “effective against multiple strains or variants as opposed to just one,” explained the medical site verywell health.
Rajesh Gandhi, MD, an infectious diseases physician at Massachusetts General Hospital and chair of the HIV Medicine Association, told verywell health, “The mRNA platform makes it easy to develop vaccines against variants because it just requires an update to the coding sequences in the mRNA that code for the variant.”
HIV has at least 16 known variants today, says LGBTQNation.
If you wish to know more about the mRNA technology, please read here and here.
The study is reportedly a collaboration among ModernaTX, Inc., The University of Texas at San Antonio, George Washington University, Fred Hutchinson Cancer Research Center, and Emory University. To read more about the Phase I trial, read here.
Clinical trials for previously formulated HIV vaccines were unsuccessful. One such trial was conducted in Thailand during the 2000s. This vaccine “used inactivated forms of the virus” and the findings unfortunately revealed that it “actually increase people’s risk of catching HIV rather than preventing infections.”
As of 2020, approximately 37.6 million people around the world are living with HIV, HIV.gov reports. Out of this figure, 35.9 million were adults while 1.7 million were children who are less than 15 years of age.









Let’s hope this trial is successful!!!!
This is VERY BAD NEWS. Even the creator of the process has stated that mRNA should *never* be used as a “vaccine,” as it shreds the DNA of the host cells. For uninfected people, this is the equivalent of committing suicide by shutting down the immune system, leading the body to attack healthy cells as if they were infected… which means a simple cold can be a death sentence, just like in full-blown AIDS.
And your medical degree would be from??????
Reality and intelligent research. Where’s yours from? Some Cabal-funded propaganda machine?
Nice! Now let’s bring it back to reality and talk about the drug companies. Not only are they making a killing off HIV+ people who need the drugs, now they’ve successfully tapped the HIV- market with PrEP. A successful vaccine would essentially mean the end of HIV…which would also eliminate the need for the all drugs. Ain’t gonna happen! Too much $$$ in keeping people sick and dependent.
Have you considered the bigger picture? This is a global extermination program, as a part of UN Agenda 21 and UN Agenda 2030. HIV just wasn’t effective enough, so they tried COVID. COVID has a 99.7% survival rate, so they use mRNA “vaccines” to do the job… and people are gullible enough to take mRNA “vaccines” for which the industry and government accept NO LEGAL LIABILITY.